What Is The Name Given To The Group 0 Elements
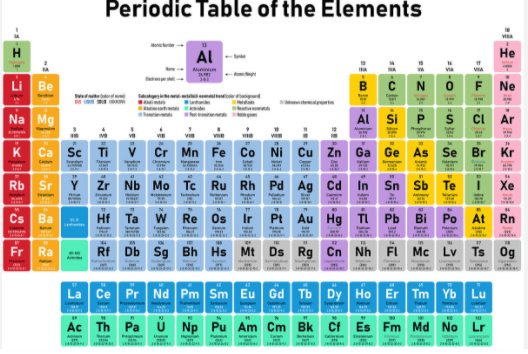
The periodic table is a fundamental tool in chemistry, used to organize and classify the elements based on their physical and chemical properties. The table consists of numerous groups that share similar characteristics, making it easier for scientists to predict the behavior of various elements.
One such group is Group 0, also known as the noble gases. Comprising helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn), these elements are called noble gases because they are resistant to reacting chemically with other substances under normal conditions. This unique property has led to numerous applications in various industries, including lighting, medical imaging, and nuclear power generation.
In this article, we will explore the composition of Group 0 elements, their properties, history of discovery and naming, uses in modern-day society and future research prospects for these fascinating elements.
What are Groups on the Periodic Table?
The groups on the periodic table represent vertical columns that organize elements based on their shared chemical and physical properties, allowing for easier identification of trends and patterns in the behavior of atoms. This classification of elements is a fundamental aspect of chemistry since it enables chemists to predict how atoms will react with each other and with other molecules.
Each group has its unique characteristics that distinguish it from others, mainly due to the valency electrons’ distribution in the outermost shell. These electrons significantly determine an element’s reactivity, ionization energy, electronegativity, melting point, boiling point, and other physical properties. Therefore, understanding trends in group properties is crucial for predicting an element’s behavior under different conditions and developing new materials for various applications.
The Composition of Group 0
Comprising of noble gases, this group consists of elements that are highly unreactive due to their stable electron configurations.
The physical properties of these elements include low boiling and melting points, as well as high densities and thermal conductivities.
These gases possess a lack of color, taste, and odor at room temperature and pressure. They are also monatomic in nature, meaning they exist as individual atoms rather than molecules.
Due to their filled outermost energy levels, noble gases do not readily form compounds with other elements, making them chemically inert or non-reactive.
However, some exceptions exist where certain compounds have been synthesized under specific conditions such as high pressures or temperatures.
Despite their unreactivity, noble gases find extensive use in various applications such as lighting technologies and cryogenics.
Properties of Group 0 Elements
Noble gases possess unique physical and chemical properties due to their stable electron configurations, making them highly unreactive and monatomic in nature. These elements have a full valence shell of electrons, which makes them chemically inert and resistant to forming bonds with other elements.
In terms of physical properties, noble gases are colorless, odorless, tasteless, and non-toxic gases that exist at room temperature as monoatomic molecules. They have low boiling points and melting points, indicating weak intermolecular forces between the individual atoms.
Due to their lack of reactivity, they are often used in lighting technology (neon signs), cryogenics (helium cooling), deep-sea diving (helix mixture), and medical applications (xenon anesthesia). Despite their apparent stability and inertness, some noble gases can still form compounds under extreme conditions or when exposed to highly reactive species such as fluorine or oxygen.
Overall, the unique combination of chemical reactivity and physical properties make noble gases fascinating elements for research purposes and various practical applications.
The History of Noble Gases
Throughout the centuries, these elements have been observed and studied by scientists, with their unique properties fascinating researchers and leading to groundbreaking discoveries.
The history of noble gases is a long one, stretching back to the 18th century when Henry Cavendish discovered a gas that was remarkably unreactive. This gas would later be identified as hydrogen, but it sparked interest in other gases that were similarly inert.
In the early 20th century, chemists finally identified a group of elements that shared this property: helium, neon, argon, krypton, xenon, and radon. These elements were dubbed the ‘noble gases’ due to their lack of reactivity with other substances.
Since then, they have found numerous applications in industry and medical use due to their chemical stability and unique properties such as luminescence and high ionization potential.
Despite being rare in Earth’s atmosphere, they are crucial for many technological advancements today.
Discovery of Helium
In 1868, French astronomer Jules Janssen and English astronomer Joseph Norman Lockyer independently discovered a new spectral line during a solar eclipse which later led to the discovery of helium. This element was named after the Greek word for sun, “Helios, “because it was first observed in the spectrum of sunlight. Helium is a colorless and odorless gas that is lighter than air and is found in small amounts in the Earth’s atmosphere. It has unique properties such as being non-flammable, non-reactive, and has one of the lowest boiling points of any element. These properties make helium useful for many commercial applications such as welding, cooling nuclear reactors, and filling balloons. The discovery of helium not only expanded our knowledge of elements but also gave us insight into the properties that make this element so versatile.
Discovery of Neon
Following the discovery of Helium, another noble gas was discovered in 1898 by Sir William Ramsay and Morris W. Travers, which they named Neon due to its bright orange-red glow when excited with electricity.
They had isolated this rare gas from liquid air using fractional distillation and observed its unique properties, such as its inertness and lack of reactivity with other elements.
The discovery of neon had a significant impact on the scientific community’s understanding of noble gases’ behavior and properties.
Neon has since been used in various applications such as lighting, advertising signs, and cryogenics due to its unique physical characteristics.
This discovery method paved the way for further exploration into other noble gases’ properties and their potential uses in different fields of study.
Discovery of Argon
The isolation of a new noble gas was achieved by Lord Rayleigh and William Ramsay in 1894, which they discovered to be present in the Earth’s atmosphere and named it Argon. This discovery was made possible through the use of various isolation methods such as fractional distillation of liquid air and chemical reactions with other elements. Argon is a colorless, odorless, and tasteless gas that has a density approximately twice that of air. It is non-reactive under normal conditions and has a boiling point of -185.7°C, making it one of the coldest liquids known to man. Its physical properties make it useful in many industrial applications, including welding and lighting. Overall, the discovery of Argon opened up new avenues for research on noble gases and their unique properties that continue to fascinate scientists today.
| Physical Property | Value |
|---|---|
| Boiling point | -185.7°C |
| Density | 1.784 g/L |
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
Note: Values are at standard temperature (0°C) and pressure (1 ATM).
Discovery of Krypton
Lord Rayleigh and William Ramsay continued their research on noble gases after the discovery of Argon, which led to the isolation of Krypton in 1898 through similar methods.
Krypton is a colorless, odorless, tasteless gas that is located in group 18 (or group 0) of the periodic table. It has a full outer shell of electrons, making it stable and unreactive.
Although krypton is not very abundant in Earth’s atmosphere, it can be found in small amounts along with other noble gases such as neon and xenon.
Krypton’s uses are limited due to its scarcity; however, it does have some practical applications such as being used as a filling gas for fluorescent lamps and photographic flash bulbs.
In terms of comparison to other noble gases, krypton has a higher boiling point than both neon and helium but lower than xenon. Additionally, its atomic radius falls between those two elements as well.
Discovery of Xenon
Xenon, a noble gas discovered by Sir William Ramsay and Morris Travers in 1898, has various practical applications despite its rarity in Earth’s atmosphere. Its unique properties have been explored for decades, leading to the discovery of its usefulness in different industries.
Here are the three main areas where xenon is applied:
- Lighting: Xenon lamps are used in high-intensity discharge (HID) lights commonly found in sports stadiums, car headlights, and movie projectors. These lamps produce a bright white light that lasts longer than conventional bulbs.
- Medical Imaging: Xenon gas is used as a contrast agent in medical imaging procedures such as computed tomography (CT) scans and magnetic resonance imaging (MRI). When this gas is breathed by patients, it enhances images of their lungs or brain, making it easier for doctors to diagnose illnesses.
- Research: Xenon’s unique properties make it an ideal element for scientific research purposes. It can be used as a cryogen or refrigerant because it remains gaseous at extremely low temperatures.
Despite being rare on Earth’s surface, xenon has proven to be useful across multiple industries due to its unique properties. Its applications range from lighting to medical imaging and scientific research. As we explore further into the world of chemistry and physics, who knows what other uses we might find for this elusive noble gas?
Discovery of Radon
In 1899, Friedrich Ernst Dorn discovered a radioactive gas that was later named radon.
Radon is a noble gas and belongs to Group 18 of the periodic table.
It is colorless, odorless, and tasteless.
Radon is formed by the decay of uranium or thorium in soil, rocks, and water.
The properties of radon make it useful for various applications such as radiation therapy for cancer patients and in geologic studies.
However, prolonged exposure to high levels of radon can lead to health problems such as lung cancer.
In fact, radon exposure is responsible for about 21,000 lung cancer deaths each year in the United States alone.
Therefore, it is important to ensure that levels of radon are kept within safe limits in homes and other buildings where people spend extended periods of time.
Read also: How To Clean A Filter On An Outside Oil Tank
Naming of Group 0 Elements
The Group 0 elements, which are also known as the noble gases, are notable for their stable electronic configurations and inertness towards chemical reactions.
The name ‘noble’ was given to these elements because of their perceived superiority and lack of reactivity with other substances.
In addition to the noble gases, this group has also been referred to as the inert gases or rare gases.
Origin of the Name “Noble Gases”
Derived from the Greek word ‘Argos’ meaning ‘inactive,’ the group 0 elements are commonly known as noble gases due to their general lack of reactivity. This term was first coined by Sir William Ramsay in 1898, after he discovered these inert element through his experiments on air samples.
The name noble was used to reflect their unique properties and distinguished them from other reactive elements in the periodic table. These gases are characterized by stable electron configurations that make them highly unreactive, which is why they were previously considered to be chemically inactive.
However, recent studies have shown that some noble gases can react under certain conditions and form compounds with other elements, challenging the traditional view of them being entirely inert. Despite this new understanding, the name noble gases remains an important part of scientific terminology and serves as a reminder of their extraordinary qualities.
Other Names for Group 0
One alternative term used to refer to the group 0 elements is the ‘inert gases’, acknowledging their lack of reactivity.
These elements are also commonly referred to as ‘noble gases’ due to their perceived superiority over other elements.
However, it should be noted that there are some differences between Group 0 and other groups on the periodic table.
For instance, while most elements readily bond with other atoms to form compounds, Group 0 elements do not.
This unique property has led them to be categorized as a separate class of elements altogether.
Other names for Group 0 include ‘rare gases’ and ‘helium family’.
Uses of Group 0 Elements
Utilization of group 0 elements in various industries and technologies is attributed to their stable electronic configuration, rendering them unreactive under normal conditions. These elements are commonly used in cryogenics applications, where they are used as coolants due to their ability to liquefy at extremely low temperatures.
In addition, group 0 elements like helium and xenon are essential components of medical imaging technologies such as magnetic resonance imaging (MRI) machines, positron emission tomography (PET) scanners, and gas chromatography detectors.
Helium is also widely used for arc welding and leak detection processes due to its inertness and high thermal conductivity. With their unique properties, the uses of group 0 elements continue to expand across different fields, providing valuable contributions that enhance our understanding of science and technology.
Future Research and Applications
Exploration of the potential applications and properties of these unreactive elements continues to pave the way for groundbreaking innovations, with their inertness likened to a blank canvas waiting for creative minds to paint with novel ideas. Potential future discoveries in this field include the use of helium as a coolant in nuclear reactors, xenon as an anesthetic agent, and neon as a plasma source in lighting technology. Additionally, practical applications are being investigated such as using argon gas to preserve historical documents and artwork due to its non-reactive nature. The table below shows some potential future discoveries and practical applications of group 0 elements.
| Group 0 Element | Potential Future Discoveries | Practical Applications |
|---|---|---|
| Helium | Coolant in nuclear reactors | Balloons, airships, MRI machines |
| Neon | Plasma source in lighting technology | Neon signs |
| Argon | Insulation material in windows and walls | Preserving historical documents and artwork |
| Xenon | Anesthetic agent for medical procedures | Lighting technology |
The possibilities for these elements seem endless, with researchers continuously exploring new ways to utilize their unique properties. As new technologies emerge and industries evolve, it is certain that group 0 elements will play an important role in shaping our future world.
Frequently Asked Questions
What are the physical and chemical properties of group 0 elements?
Group 0 elements, also known as noble gases, have unique physical and chemical properties that make them useful in various applications. Their inertness and stability make them ideal for use in lighting, welding, and cryogenics. In comparison to other elements, noble gases have low boiling points and cannot easily form compounds with other elements due to their full valence shells.
Why are group 0 elements also called noble gases?
Like a fortress protected by its walls, group 0 elements are also known as noble gases due to their full outer shell configuration. This makes them stable and unreactive, unlike other groups on the periodic table that seek to gain or lose electrons to achieve stability.
How do group 0 elements react with other elements?
Group 0 elements, also known as noble gases, have a low reactivity due to their filled outer electron shells. Possible research areas related to their reactivity include exploring potential compounds and reactions with extreme conditions. Experimental techniques used to study group 0 element interactions include spectroscopy and computational modeling.
What are the industrial uses of group 0 elements?
Group 0 elements, also known as noble gases, have unique properties that make them valuable in various industrial applications. Helium is used in cryogenics and welding, neon is utilized in lighting, and argon serves as a shielding gas during welding. Their inertness and stability also make them ideal for medical applications and environmental impact studies.
What are the potential health hazards associated with exposure to group 0 elements?
Occupational exposure to group 0 elements, such as helium and neon, is generally considered safe due to their inert nature. However, environmental pollution from industrial processes involving these elements may have potential health hazards for humans and the environment.
Conclusion
Groups on the periodic table are vertical columns that represent elements with similar chemical and physical properties.
Group 0, also known as the noble gases, is composed of helium, neon, argon, krypton, xenon, and radon. These elements are all odorless, colorless, and have low reactivity due to their full outer electron shells.
The history of noble gases dates back to the late 1800s when scientists began studying their unique characteristics. Helium was discovered during a solar eclipse in 1868 by French astronomer Jules Janssen. Radon was later discovered in 1899 by German physicist Friedrich Ernst Dorn.
The name ‘noble gases ‘comes from their reluctance to react with other elements.
Group 0 elements have various uses such as in lighting technology (neon lights), welding (argon), medical imaging (xenon), and nuclear medicine (radon). Ongoing research focuses on using noble gases for new applications such as gas separation and purification processes.
In conclusion, what is the name given to Group 0 elements? The answer is the noble gases. These fascinating elements have a rich history and interesting properties that make them valuable for numerous applications today and in future research. As we continue to explore their potential uses, who knows what exciting discoveries we will uncover next?